Health Canada approves Dysport to treat kids with cerebral palsy
Health Canada has approved Dysport (abobotulinumtoxinA) for the treatment of leg spasticity in children and teens ages 2 and older, including those with cerebral palsy.
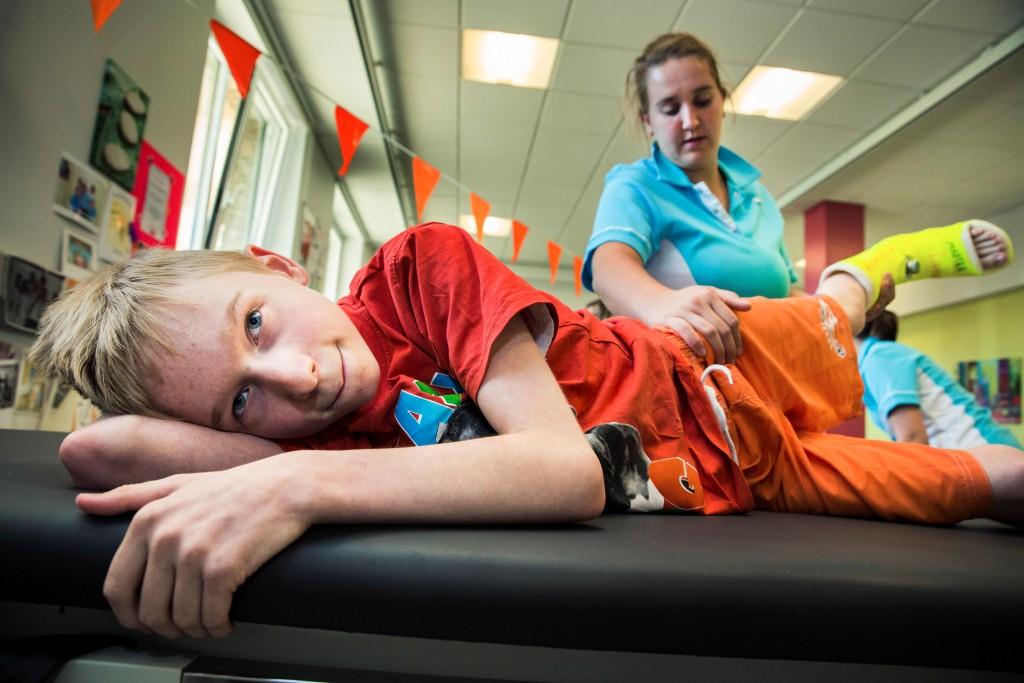
Six months ago, 13-year-old Georges Vermast rode her bike for the first time in her life – from her home in the coastal city of Oostduinkerke straight to the beach. Even though she wasn’t riding a real bike but a tricycle, it was a remarkable achievement and a demonstration of how endurance and devotion can eventually pay off. A year before, Georges had been bound to her wheelchair, believing she would never be able to ride a bike or even walk independently. Dieter Telemans photo, Children’s Rehabilitation Department of UZ Leuven Pellenberg
By Magdalena Kegel, Cerebral Palsy News Today January 10, 2018
The treatment, which reduces spasticity by blocking nerve signaling, was previously shown to effectively improve muscle tone and movement ability in children. It also improved measures of general health.
“With the Health Canada approval of Dysport Therapeutic, children with cerebral palsy and their parents can look forward to another therapy which improves stiffness and movement that are hallmarks of cerebral palsy,” Janice Bushfield, executive director of the Cerebral Palsy Association in Alberta, said in a press release.
“It is wonderful news for this community since, when it comes to medication and cerebral palsy, one size does not fit all,” Bushfield added.
Dysport, developed by Ipsen, was approved in the U.S. in 2016 for leg spasticity in this pediatric patient group.
An earlier Phase 3 trial (NCT01249417) and its extension (NCT01251380) demonstrated that Dysport improved walking and balance, decreased falling and tripping, and improved endurance in children and teens ages 2 to 17.
The studies included patients who could walk, but had so-called equinus foot deformity caused by cerebral palsy. Patients also received other treatments during the clinical trials. The Phase 3 extension study showed that repeated injections of the medication were safe.
“The stiffness that accompanies pediatric lower limb spasticity can be painful and disabling for children who suffer from movement disorders, including cerebral palsy,” said Michael Shevell, chair of the Department of Pediatrics at McGill University in Canada.
“Additional choices for this patient population are required since current regimens may not be suitable for every child and treatment needs to be individualized,” he said.
The majority of patients with cerebral palsy have leg spasticity, which makes walking more difficult in 80 percent of children with the condition. Spasticity is often caused by damage to the spinal cord or parts of the brain that control movement, making some neurons overactive.
Dysport prevents spasticity by blocking signals from these neurons.
“The approval of Dysport Therapeutic in this pediatric indication demonstrates Ipsen Canada’s ongoing commitment to spasticity and neuromuscular disorders,” said Paul Reider, general manager for Ipsen Canada.
“We are proud to play a role in improving the lives of families who are affected by pediatric lower limb spasticity by continuing to expand the indications for this important treatment option,” Reider added.
Source Cerebral Palsy News Today
| References |
Safety and Efficacy of Repeat Open-Label Abobotulinumtoxin: A Treatment in Pediatric Cerebral Palsy, Mauricio R Delgado MD, Marcin Bonikowski MD, Jorge Carranza MD, Edward Dabrowski MD, Dennis Matthews MD, Barry Russman MD, Ann Tilton MD, Juan Carlos Velez MD, Anne-Sophie Grandoulier MSc, and Philippe Picaut PharmD. J Child Neurol. 2017 November; 32(13): 1058–1064. Published online 2017 September 15. doi: 10.1177/0883073817729918
AbobotulinumtoxinA: A Review in Pediatric Lower Limb Spasticity, Syed YY. Paediatr Drugs. 2017 Aug;19(4):367-373. doi: 10.1007/s40272-017-0242-4.
AbobotulinumtoxinA for Equinus Foot Deformity in Cerebral Palsy: A Randomized Controlled Trial, Mauricio R. Delgado, Ann Tilton, Barry Russman, Oscar Benavides, Marcin Bonikowski, Jorge Carranza, Edward Dabrowski, Nigar Dursun, Mark Gormley, Marek Jozwiak, Dennis Matthews, Iwona Maciag-Tymecka, Ece Unlu, Emmanuel Pham, Anissa Tse, Philippe Picaut. Pediatrics Jan 2016, peds.2015-2830; DOI: 10.1542/peds.2015-2830
Botulinum toxin (Dysport) treatment of the spastic gastrocnemius muscle in children with cerebral palsy: a randomized trial comparing two injection volumes, Hu GC, Chuang YC, Liu JP, Chien KL, Chen YM, Chen YF. Clin Rehabil. 2009 Jan;23(1):64-71. doi: 10.1177/0269215508097861.
Treatment of paediatric cerebral palsy with Dysport, Ubhi T. Hosp Med. 2000 Oct;61(10):718-21.
Treatment with Botulinum toxin A in a total population of children with cerebral palsy – a retrospective cohort registry study, Franzén M, Hägglund G, Alriksson-Schmidt A. BMC Musculoskelet Disord. 2017 Dec 11;18(1):520. doi: 10.1186/s12891-017-1880-y. PDF
Also see
Ipsen Announces Health Canada Approval of DYSPORT THERAPEUTIC™ (abobotulinumtoxinA) for the Treatment of Patients with Cervical Dystonia and Adult Upper Limb Spasticity Cision Newswire
Ipsen Biopharmaceuticals Canada Inc. Announces Health Canada Approval of DYSPORT THERAPEUTIC™ (abobotulinumtoxinA) for the Treatment of Lower Limb Spasticity in Children Aged Two and Older Marketwired
Dysport Safe, Effective in Kids with CP Even When Used Repeatedly, Researchers Find Cerebral Palsy News
Additional Positive Trial Results Reported for Dysport in Cerebral Palsy with Lower Limb Spasticity Cerebral Palsy News Today
Dysport phase III study results in children with cerebral palsy with lower limb spasticity published in Pediatrics Medical Life Sciences News
FDA Clears Dysport for Lower-Limb Spasticity in Kids Medscape Medical News
Botox Treatment for Cerebral Palsy Depends on Age, Sex, Gross Motor Function, Study Shows Cerebral Palsy News Today
