New treatment to regain hand and arm function after spinal cord injury
Using physical therapy combined with a noninvasive method of stimulating nerve cells in the spinal cord, University of Washington researchers helped six Seattle area participants regain some hand and arm mobility. That increased mobility lasted at least three to six months after treatment had ended. The research team published these findings Jan. 5 in the journal IEEE Transactions on Neural Systems and Rehabilitation Engineering.
| New hope for people with spinal cord injuries. UW (University of Washington) Youtube Jan 12, 2021 |
Sarah McQuate UW News, University of Washington January 12, 2021
Almost 18,000 Americans experience traumatic spinal cord injuries every year. Many of these people are unable to use their hands and arms and can’t do everyday tasks such as eating, grooming or drinking water without help.
“We use our hands for everything — eating, brushing our teeth, buttoning a shirt. Spinal cord injury patients rate regaining hand function as the absolute first priority for treatment. It is five to six times more important than anything else that they ask for help on,” said lead author Dr. Fatma Inanici, a UW senior postdoctoral researcher in electrical and computer engineering who completed this research as a doctoral student of rehabilitation medicine in the UW School of Medicine.
“At the beginning of our study,” Inanici said, “I didn’t expect such an immediate response starting from the very first stimulation session. As a rehabilitation physician, my experience was that there was always a limit to how much people would recover. But now it looks like that’s changing. It’s so rewarding to see these results.”

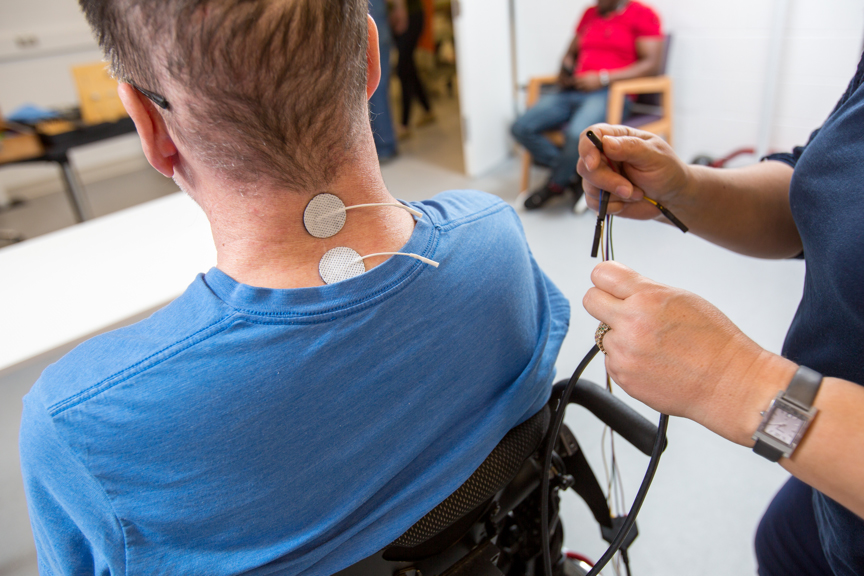
Caption: Using physical therapy combined with a noninvasive method of stimulating nerve cells in the spinal cord, University of Washington researchers helped six Seattle area participants regain some hand and arm mobility. Here, Dr. Fatma Inanici (green shirt), a UW senior postdoctoral researcher in electrical and computer engineering, applies small patches that will deliver electrical currents to the injured area on a participant’s neck. These patches stick to a participant’s skin like a Band-Aid. Marcus Donner, Center for Neurtechnology, University of Washington
After a spinal cord injury, many patients do physical therapy to help them attempt to regain mobility. Recently, a series of studies have shown that implanting a stimulator to deliver electric current to a damaged spinal cord could help paralyzed patients walk again.
The UW team, composed of researchers from the Center for Neurotechnology, combined stimulation with standard physical therapy exercises, but the stimulation doesn’t require surgery. Instead, it involves small patches that stick to a participant’s skin like a Band-Aid. These patches are placed around the injured area on the back of the neck where they deliver electrical pulses.
The researchers recruited six people with chronic spinal cord injuries. All participants had been injured for at least a year and a half. Some participants couldn’t wiggle their fingers or thumbs while others had some mobility at the beginning of the study.
To explore the viability of using the skin-surface stimulation method, the researchers designed a five-month training program. For the first month, the researchers monitored participants’ baseline limb movements each week. Then for the second month, the team put participants through intensive physical therapy training, three times a week for two hours at a time. For the third month, participants continued physical therapy training but with stimulation added.
“We turned on the device, but they continued doing the exact same exercises they did the previous month, progressing to slightly more difficult versions if they improved,” Inanici said.

| Using physical therapy combined with a noninvasive method of stimulating nerve cells in the spinal cord, University of Washington researchers helped six Seattle area participants regain some hand and arm mobility. Participants progressed to more difficult versions of the training exercises (for example, going from picking up a ping pong ball to picking up a tiny bead, shown here) as they improved. Marcus Donner, Center for Neurotechnology |
For the last two months of the study, participants were divided into two categories: Participants with less severe injuries received another month of training alone and then a month of training plus stimulation. Patients with more severe injuries received the opposite — training and stimulation first, followed by only training second.

| To explore the viability of using the noninvasive stimulation method, the researchers designed a five-month training program that included month-long regimens of training alone or training with stimulation. Inanici et. al, IEEE Transactions on Neural Systems and Rehabilitation Engineering |
While some participants regained some hand function during training alone, all six saw improvements when stimulation was combined with training.
“Both people who had no hand movement at the beginning of the study started moving their hands again during stimulation, and were able to produce a measurable force between their fingers and thumb,” said senior author Chet Moritz, a UW associate professor of electrical and computer engineering, rehabilitation medicine and physiology and biophysics. “That’s a dramatic change, to go from being completely paralyzed below the wrists down to moving your hands at will.”
Transcutaneous stimulation in the Moritz Lab at the University of Washington. Matt Hagen
In addition, some participants noticed other improvements, including a more normal heart rate and better regulation of body temperature and bladder function.
The team followed up with participants for up to six months after training and found that these improvements remained, despite no more stimulation.
“We think these stimulators bring the nerves that make your muscles contract very close to being active. They don’t actually cause the muscle to move, but they get it ready to move. It’s primed, like the sprinter at the start of a race,” said Moritz, who is also the co-director of the Center for Neurotechnology. “Then when someone with a spinal cord injury wants to move, the few connections that might have been spared around the injury are enough to cause those muscles to contract.”
The research is moving toward helping people in the clinic. The results of this study have already informed the design of an international multi-site clinical trial that will be co-led by Moritz. One of the lead sites will be at the UW.
“We’re seeing a common theme across universities — stimulating the spinal cord electrically is making people better,” said Moritz, who also holds the CJ and Elizabeth Hwang professorship in electrical and computer engineering. “But it does take motivation. The stimulator helps you do the exercises, and the exercises help you get stronger, but the improvements are incremental. Over time, however, they add up into something that’s really astounding.”
Lorie Brighton, a research scientist at the UW; Soshi Samejima, a UW doctoral student in rehabilitation medicine; and Dr. Christoph Hofstetter, an associate professor of neurological surgery in the UW School of Medicine, are co-authors on this paper. This research was funded by the Center for Neurotechnology, the Washington State Spinal Cord Injury Consortium and the Christopher and Dana Reeve Foundation.
| For more information, contact Inanici at finanici@uw.edu and Moritz at ctmoritz@uw.edu. Grant number: EEC-1028725 |
![]() Source University of Washington
Source University of Washington
| References |
Transcutaneous spinal cord stimulation restores hand and arm function after spinal cord injury, Inanici F, Brighton LN, Samejima S, Hofstetter CP, Moritz CT. IEEE Trans Neural Syst Rehabil Eng. 2021 Jan 5;PP. doi: 10.1109/TNSRE.2021.3049133. Epub ahead of print. PDF
A Novel Electromagnetic-Neurobiologic Interface for Functional Animation of Dormant Motor Nerve Roots in Spinal Cord Injury via Neuromodulation, Shah J, Smith RH, Al-Gharaibeh A. Front Surg. 2020 Jan 10;6:73. doi: 10.3389/fsurg.2019.00073. Full text
Transcutaneous Electrical Spinal Stimulation Promotes Long-Term Recovery of Upper Extremity Function in Chronic Tetraplegia, Inanici F, Samejima S, Gad P, Edgerton VR, Hofstetter CP, Moritz CT. IEEE Trans Neural Syst Rehabil Eng. 2018 Jun;26(6):1272-1278. doi: 10.1109/TNSRE.2018.2834339. Full text
Non-Invasive Activation of Cervical Spinal Networks after Severe Paralysis, Gad P, Lee S, Terrafranca N, Zhong H, Turner A, Gerasimenko Y, Edgerton VR. J Neurotrauma. 2018 Sep 15;35(18):2145-2158. doi: 10.1089/neu.2017.5461. Full text
Noninvasive Reactivation of Motor Descending Control after Paralysis, Gerasimenko YP, Lu DC, Modaber M, Zdunowski S, Gad P, Sayenko DG, Morikawa E, Haakana P, Ferguson AR, Roy RR, Edgerton VR. J Neurotrauma. 2015 Dec 15;32(24):1968-80. doi: 10.1089/neu.2015.4008. Epub 2015 Aug 20. Full text
Therapeutic Stimulation for Restoration of Function After Spinal Cord Injury, Ievins A, Moritz CT. Physiology (Bethesda). 2017 Sep;32(5):391-398. doi: 10.1152/physiol.00010.2017. Full text
Also see
Three people with spinal-cord injuries regain control of their leg muscles Nature
ONWARD Announces First Patient Enrolled in the Up-LIFT Pivotal Trial Assessing Safety and Effectiveness of ARC Therapy for Spinal Cord Injury Business Wire
