Wearable sensors characterize CP gait
Inertial measurement units (IMUs) facilitate the creation of a gait analysis system that is portable and suited for use in the clinic. Research suggests IMUs can be used to measure clinically important gait metrics in children with cerebral palsy, which may improve patient outcomes.

Experimental setup: a single subject getting ready for full-factorial trials on a tredmill. Department of Biostatistics, Virginia Commonwealth University, Richmond, VA. Sensors
By Mahmoud El-Gohary PhD, Sean Pearson BS, Paul Vasilyev BS, James McNames PhD, and James Carollo PhD PE, Lower Extremity Review January 2017
The need to characterize human movement has consistently driven researchers to develop improved motion analysis systems. The primary measurement challenge is to design devices and algorithms that can accurately monitor movement regardless of the activity and testing location. Movement has typically been captured using optical, mechanical, or inertial sensing instruments.
Although each system offers its own advantages, they all have unique limitations that may distract clinicians from their core responsibilities when deployed in a clinical environment. The current standard for measuring movement in a clinical environment involves instruments that have varying degrees of accuracy and complexity and may be administered differently, depending on the user’s biomechanical expertise.
Instrumented gait analysis (IGA) is the most common quantitative method used for clinical gait assessment. The technique is laboratory based and most often uses optical motion capture to characterize the kinematics of human movement. Although these systems are the industry gold standard, they are costly, cumbersome, and may suffer from optical occlusion, which occurs when objects interrupt the line of sight between the markers and video cameras. Because they require a dedicated motion laboratory with specialists to collect, process, and interpret movement data, they are not easily integrated into the typical outpatient clinic.
Mechanical motion capture systems such as electrogoniometers can provide precise and accurate position estimates for a single joint. However, they can be cumbersome when measuring different joints, axes of rotation, or bilateral movement patterns; are unnecessarily complex for monitoring daily activities; and can accommodate only a limited range of motion.
Inertial sensor-based gait systems could provide clinicians with significantly more useful information than a simple activity monitor or periodic video of patients’ gait.
Inertial sensors have been widely used to overcome the limitations of other motion capture systems. Inertial measurement systems continue to change rapidly, and there have been significant advances in reducing their cost and size in the last few years. A typical inertial measurement unit includes accelerometers to measure the translational acceleration in addition to gravity, gyroscopes to measure angular velocities, and magnetometers to measure the earth magnetic fields. The sensors can be attached to various body segments, and the detected movement can be continuously recorded while the wearer performs normal daily activities.
Sponsored by a grant from the National Institute of Neurological Disorders and Stroke, we have developed a wearable system with IMUs to characterize gait in children with cerebral palsy (CP). The system provides joint kinematics and temporal-spatial measures of gait, similar to those obtained by optical motion capture systems, at a fraction of the cost and with much more simplicity, automation, and ease of use.
| Background |
CP is a group of permanent disorders affecting the development of movement and posture and resulting in mobility limitations. The most common observed symptoms of CP are lack of muscle coordination during voluntary movements, exaggerated reflexes (spasticity), and muscle weakness. Severity of the disease depends on the area of the brain affected and the extent of the injury; consequently, associated gait abnormalities are unique to each individual.
While each person with CP has a characteristic gait, several typical patterns are often displayed. These include jump knee, crouch knee, stiff knee, knee recurvatum, and varying types of equinus gait. Jump knee gait is described as a toe-walking pattern with increased knee flexion in the early stance phase of gait, followed by a rapid knee extension in late stance, while the pelvis is tilted anteriorly.
Crouch gait is a pattern with excessive hip and knee flexion during both the stance and swing phases, with the ankle often excessively dorsiflexed in stance. Stiff knee gait indicates a gait pattern with insufficient knee flexion during swing, leading to reduced foot clearance during swing and distinct compensatory movements. Knee recurvatum is characterized by knee hyperextension in mid- or terminal stance, often related to a forward body position or an exaggerated plantar flexion/knee extension couple.
Equinus gait is another type of toe-walking, characterized by excessive ankle plantar flexion during stance, but without the dynamic knee motion seen in jump knee gait. This pattern of toe-walking has been more recently referred to as “true equinus,” to distinguish it from a toe-walking pattern that may be similar in appearance, known as “apparent equinus.” In apparent equinus, toe-walking is the result of excessive knee flexion in stance with a relatively neutral ankle position, rather than the excessive plantar flexion associated with true equinus. Goldstein and Harper, among others, have stated that failing to distinguish between true equinus and apparent equinus is the most common and potentially damaging mistake in observational gait analysis.
IGA is an essential tool for distinguishing among the gait patterns associated with CP, and any measurement system used to characterize gait performance in individuals with CP should provide clinicians with the quantitative information necessary to identify them accurately.
The current standard of expert care for children with gait abnormalities is to obtain IGA pre- and post-treatment interventions. In addition to measuring static joint ranges of motion, a full IGA includes at least kinematic assessment of the pelvis and lower extremities during walking to characterize joint angles and quantify displacement of body segments. Despite the importance of this type of assessment, IGA systems are not available in most physical therapy clinics, and consequently are often not even considered for adults with CP, very young children, and individuals with gait abnormalities other than CP who could benefit from clinical gait analysis.
We developed an inertial system that can provide measures of joint kinematics and gait similar to those provided by IGA, but is cheaper, more efficient, and easier to use in the clinic. The system uses lightweight, wearable IMUs, which stay securely attached to the body, allowing users to perform unrestricted movements. It provides objective measures of 3D gait kinematics and temporal-spatial parameters (walking speed, cadence, step times, and step lengths) describing movement patterns and the time-varying nature of motion at the hip, knee, and ankle joints in the sagittal, frontal, and horizontal planes. The system also characterizes symmetry and variability of temporal events between each leg during walking, including swing and step duration, timing, and consistency.
| Figure 1 |

| Figure 1. An Opal wearable sensor with a Velcro strap in a docking station used to recharge the device. APDM Wearable Technologies, Portland OR. LER |
| The system |
Our inertial system uses seven wearable IMUs attached to the pelvis, femur, tibia, and foot (Figure 1). The IMUs use wireless synchronization, which is critical for measuring coordination of the hip, knee, and ankle of both sides relative to the trunk. We developed a tracking algorithm to estimate joint angles and range of motion of the lower limb during gait, using the IMU data.
The algorithm tracks 3D motion/orientation of the pelvis, and associated hip, knee, and ankle joint angles bilaterally during any bipedal task. It also provides temporal-spatial measures of gait, along with a quantitative tool to assess the critical gait events that occur at the foot, ankle, knee, or hip during a typical walking cycle. These critical events, as described by Perry, occur during specific phases of the gait cycle and are considered an essential requirement for producing a normal walking pattern. Examples include a heel-first initial contact; existence of controlled ankle plantar flexion (first rocker) and a knee flexion wave during loading response; controlled ankle dorsiflexion (second rocker) during midstance; hip extension with a trailing limb posture during terminal stance; and rapid hip flexion with synchronous ankle plantar flexion (third rocker) during preswing.
The significance of this approach is that once these critical gait events are identified, clinicians can use the IMU data to determine functional reasons for why a particular critical event is absent, altered, or delayed. Interventions can then be focused on restoring delayed, altered, or missing critical events, leading to improved walking performance. Figure 2 shows an example of hip, knee, and ankle angles during a normalized gait cycle for a typically developing girl aged 10 years. The dark green and red lines represent the average of right and left limb angles respectively across all gait cycles during a two-minute walk. The light green and red bands represent ± 1 standard deviation. The green, red, and blue columns indicate the gait phases of loading response (LR), mid/terminal stance (M/TS), and preswing (PS), respectively, and the corresponding first, second, and third rockers of the foot and ankle.
| Figure 2 |
| Figure 2. Ankle, hip, and knee joint angles quantified by the inertial system for a healthy girl aged 10 years. The angles are normalized to 100% of the gait cycle with the green, red, and blue columns indicating the phases in which critical events occur. Green and red lines represent angles of the right and left leg. LER |
| Validation and results |
Although optical systems may have limitations, they are the most common and accurate method available for tracking movement in a laboratory environment. Therefore, we used an industry standard motion capture system as a reference to evaluate the performance of our inertial tracking algorithm in children with and without CP.
The validation study was conducted at the Center for Gait and Movement Analysis (CGMA) at Children’s Hospital Colorado/University of Colorado in Denver, which is equipped with a 13-camera motion capture system. We compared the inertial metrics with those obtained from the motion capture system in five children with CP and five age-matched typically developing children (13.6 ± 4.1 years; six girls). Primary inclusion criteria of the CP group include a clinical diagnosis of cerebral palsy (spastic diplegia or hemiplegia), GMFCS (Gross Motor Function Classification System) level of I-III, aged between 7 and 21 years, able to walk continuously for two minutes, able to provide informed consent or assent, and able to follow directions and otherwise comply with testing instructions.
| Table 1 |
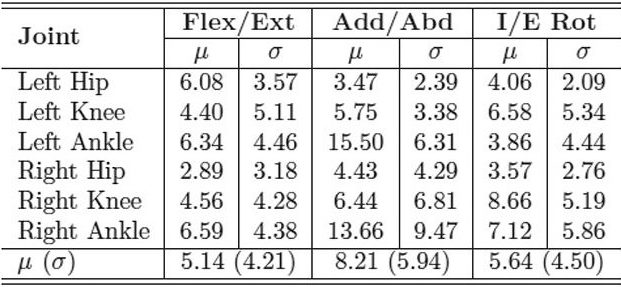
| Table 1. MAE in degrees of inertial range of motion for IMU system compared with industry standard motion capture system for the CP and control groups. LER |
| Table 2 |
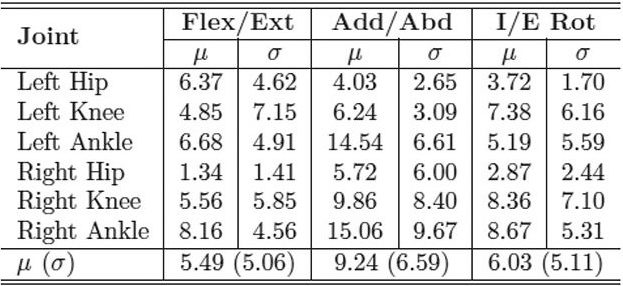
| Table 2. MAE in degrees of inertial range of motion for IMU system compared with industry standard motion capture system for the CP group. LER |
| Table 3 |
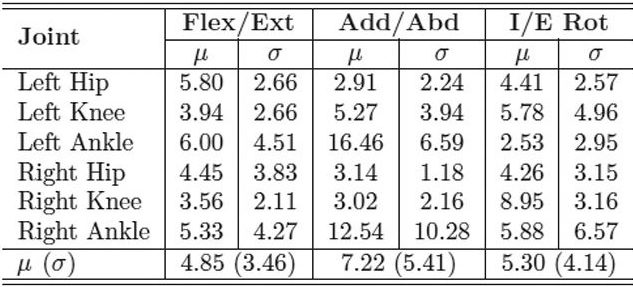
| Table 3. MAE in degrees of inertial range of motion for IMU system compared with industry standard motion capture system for the control group. LER |
With the IMUs and reflective markers attached to the pelvis, thighs, shanks, and feet, each child walked back and forth along a 40-foot walkway at a self-selected pace for two minutes. The inertial and optical data were processed after data collection to calculate the lower limb joint angles. We used the mean absolute error (MAE) to compare the joint range of motion for both gait systems. Table 1 shows the MAE for the entire study group, including both the typically developing children and those with CP. The MAE ranged from 5.1° ± 4.2° to 8.2° ± 5.9°. This is a reasonable error range compared with previous studies that used inertial sensors to track simple planar movements, which reported error ranges up to 20°. Tables 2 and 3 show the MAE for the CP and control groups separately. Despite the larger variability of gait and joint angle tracking in the CP group, the tables show comparable errors for the CP and control groups.
Despite the good agreement, we believe some of the estimation errors might be attributed to misalignment of the IMU frame relative to the anatomical axes of rotation. When the IMUs are attached to different body segments, they are not perfectly aligned with the segment main axes of rotation. Therefore, we asked the study participants to remain stationary in a neutral pose for about three seconds at the beginning of the recording to estimate this misalignment. This information is then used to realign the sensor data for analysis using matrix rotation before calculating the joint angles. While this addresses misalignment of the sensors relative to the anatomical axes, it assumes the participant can both remain stationary and adopt a truly neutral initial pose. Often individuals with CP will have joint contracture or limb-length deficiencies that prevent them from standing in this neutral pose, and we hypothesize that this contributed to the larger errors seen in this group. We are developing other analytical and procedural techniques to address this potential issue.
Another source of disagreement between the inertial and optical systems could be attributed to skin motion artifact and muscle movements, a known issue with systems that use markers attached to the body. Similarly, skin artifacts also influence the inertial sensor measurements, resulting in orientation changes in the segments. These orientation changes may produce joint angle estimates that are biomechanically unlikely, and lead to a disagreement between the systems.
| Figure 3 |
| Figure 3. Joint angles of the left (red) and right (green) lower limb of a left hemiparetic girl with CP, aged 10 years. The left foot is held in plantar flexion with a weaker dorsiflexion. Flexion of the left knee and hip is reduced during the swing phase compared with the right knee and hip. LER |
Figure 3 shows joint angles of the right (green) and left (red) lower limb of a girl aged 10 years with left hemiplegic CP, measured using the inertial system. The figure shows the left ankle is plantar flexed throughout the gait cycle relative to the right side and relative to the age- and gender-matched control participant shown in Figure 2. The child with CP also walked with reduced left knee flexion during swing.
The presence of both reduced knee flexion and excess plantar flexion in swing can lead to a foot clearance problem during limb advancement, which increases the risk of tripping and falling. In CP, lack of knee flexion in swing is an example of stiff-knee gait described above, and excess plantar flexion throughout the gait cycle is an example of “true equinus”—or, more specific to the swing phase, drop-foot gait. Figure 3 illustrates that the inertial system can detect common gait problems in a child with CP, which has important clinical significance and treatment implications.
| Conclusion |
Our findings suggest that it is feasible to use IMUs to measure normal and abnormal gait metrics that are clinically important. The system is portable and unobtrusive, and requires no lengthy setup or calibration, all of which make the system well suited for use in the clinic. The system will enable clinicians to capture patients’ movement during their unconstrained, spontaneous activities of daily life either in their home environment, which could involve fewer sensors depending on the measures of interest, or in the clinic. This would provide clinicians with significantly more useful information than a simple activity monitor or periodic video of their patients’ gait. The impact of this technology may lead to better clinical decisions that are more accessible to the disabled population than current methods of IGA.
Individuals with CP may display an array of gait patterns, depending on the severity and location of the original injury to the brain. Gait analysis with the inertial system will make it easier for clinicians to recognize these different gait patterns by quickly reviewing the kinematics of the hip, knee, and ankle joints throughout the gait cycle to detect delayed, altered, or missing critical gait events. Clinicians can then use these objective, quantitative measures to characterize their patients’ gait before and after an intervention, and to compare each patient’s information to reference data from an age-matched typically developing cohort.
The inertial-system−based analysis is not a replacement for clinical experience. Rather, it is a tool that complements clinical judgment and can help improve diagnosis and recommendations for therapeutic and alternative interventions or rehabilitation strategies. It can also facilitate the collection of clinical trial outcome measures in most outpatient clinics, rather than specialized laboratory settings. By extending the benefits of IGA to more individuals with childhood disabilities such as cerebral palsy, clinical outcomes can be continually improved and documented.
| Mahmoud El-Gohary, PhD, is a research engineer at APDM Wearable Technologies in Portland, OR. |
| Sean Pearson, BS, is a signal-processing engineer at APDM. |
| Paul Vasilyev, BS, is a signal-processing engineer at APDM. |
| James McNames, PhD, is an APDM fellow and professor and chair of the Department of Electrical and Computer Engineering at Portland State University. |
| James Carollo, PhD, PE, is director of the Center for Gait and Movement Analysis and the Musculoskeletal Research Center at Children’s Hospital Colorado and associate professor in the departments of physical medicine and rehabilitation and orthopedics at the University of Colorado Denver. |
Source Lower Extremity Review
| References |
- Strategies for clinical motion analysis based on functional decomposition of the gait cycle, Carollo JJ, Matthews M. Phys Med Rehabil Clin N Am 2002;13(4):949-977.
- Continuous monitoring of turning mobility and its association to falls and cognitive function: a pilot study, Mancini M, Schlueter H, El-Gohary M, Mattek N, Duncan C, Kaye J, Horak FB.J Gerontol A Biol Sci Med Sci 2016 Aug;71(8):1102-8. doi: 10.1093/gerona/glw019. Epub 2016 Feb 25.
- Quantifying effects of age on balance and gait with inertial sensors in community-dwelling healthy adults, Park JH, Mancini M, Carlson-Kuhta P, Nutt JG, Horak FB. Exp Gerontol. 2016 Dec 1;85:48-58. doi: 10.1016/j.exger.2016.09.018. Epub 2016 Sep 22.
- A report: the definition and classification of cerebral palsy April 2006, Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. Dev Med Child Neurol Suppl. 2007 Feb;109:8-14.
- Rehabilitation management of children with spastic diplegic cerebral palsy, Binder H, Eng GD. Arch Phys Med Rehabil. 1989 Jun;70(6):482-9.
- Gait assessment of fixed ankle-foot orthoses in children with spastic diplegia, Abel MF, Juhl GA, Vaughan CL, Damiano DL. Arch Phys Med Rehabil. 1998 Feb;79(2):126-33.
- Common gait abnormalities of the knee in cerebral palsy, Sutherland DH, Davids JR. Clin Orthop Relat Res 1993;288:139-147.
- Sagittal gait patterns in spastic diplegia, Rodda JM, Graham HK, Carson L, Galea MP, Wolfe R. J Bone Joint Surg Br. 2004 Mar;86(2):251-8.
- The role of estimating muscle-tendon lengths and velocities of the hamstrings in the evaluation and treatment of crouch gait, Arnold AS, Liu MQ, Schwartz MH, Ounpuu S, Delp SL. Gait Posture. 2006 Apr;23(3):273-81. Epub 2005 Jun 17.
- Prevalence of specific gait abnormalities in children with cerebral palsy: influence of cerebral palsy subtype, age, and previous surgery, Wren TA, Rethlefsen S, Kay RM. J Pediatr Orthop 2005;25(1):79-83.
- The role of gait analysis in treating gait abnormalities in cerebral palsy, Chang FM, Rhodes JT, Flynn KM, Carollo JJ. Orthop Clin North Am 2010;41(4):489-506.
- Management of cerebral palsy: equinus gait, Goldstein M, Harper DC. Dev Med Child Neurol 2001;43(8):563-569.
- Classification of gait patterns in spastic hemiplegia and spastic diplegia: a basis for a management algorithm, Rodda J, Graham HK. Eur J Neurol 2001;8(Suppl 5):98-108.
- Gait analysis: normal and pathological function, Perry J, Burnfield J. 1st ed. Thorofare, NJ: Slack Inc.; 1992.
- Carollo JJ, Matthews D. Quantitative assessment of gait: a systematic approach. In: Pediatric Rehabilitation: Principles and Practice, Alexander M, Matthews D, eds. 5th ed. New York: Demos Medical Publishers; 2015: 78-112. Springer
- Limitation of attitude estimation algorithms for inertial/magnetic sensors modules, Bergmann JHM, Mayagoitia RE, Smith ICH. IEEE Robotics Automation 2007;14(3):76-87. DOI: 10.1109/MRA.2007.901320
- A portable system for collecting anatomical joint angles during stair ascent: a comparison with an optical tracking device, Bergmann JHM, Mayagoitia RE, Smith ICH. Dyn Med 2009;8:3. doi: 10.1186/1476-5918-8-3
- Inertial sensor-based knee flexion/extension angle estimation, Cooper G, Sheret I, McMillan L, Siliverdis K, Sha N, Hodgins D, Kenney L, Howard D. J Biomech. 2009 Dec 11;42(16):2678-85. doi: 10.1016/j.jbiomech.2009.08.004. Epub 2009 Sep 27.
- Soft tissue artefact assessment in humeral axial rotation, Cutti AG, Paolini G, Troncossi M, Cappello A, Davalli A. Gait Posture. 2005 Apr;21(3):341-9.
| Further reading |
Quantifying Variation in Gait Features from Wearable Inertial Sensors Using Mixed Effects Models, Cresswell KG, Shin Y, Chen S. Sensors (Basel). 2017 Feb 25;17(3):466. doi: 10.3390/s17030466. Full text, PDF
Wearable and Ambient Sensors for Healthcare and Wellness Applications, Special Issue 2017 Sensors. MDPI Basel, Switzerland. ISSN 1424-8220
| 3D gait analysis with STT inertial sensors. STT inertial sensors can easily track any part of the body. Just put them on and begin your analysis! STT Systems. Youtube Jul 21, 2014 |


