Humans have salamander-like ability to regrow cartilage in joints
The process could be harnessed as a treatment for osteoarthritis.
“We believe that an understanding of this ‘salamander-like’ regenerative capacity in humans, and the critically missing components of this regulatory circuit, could provide the foundation for new approaches to repair joint tissues and possibly whole human limbs.” Virginia Byers Kraus

A black yellow spotted fire salamander. Duke University.
Sarah Avery, Duke University Health System October 8, 2019
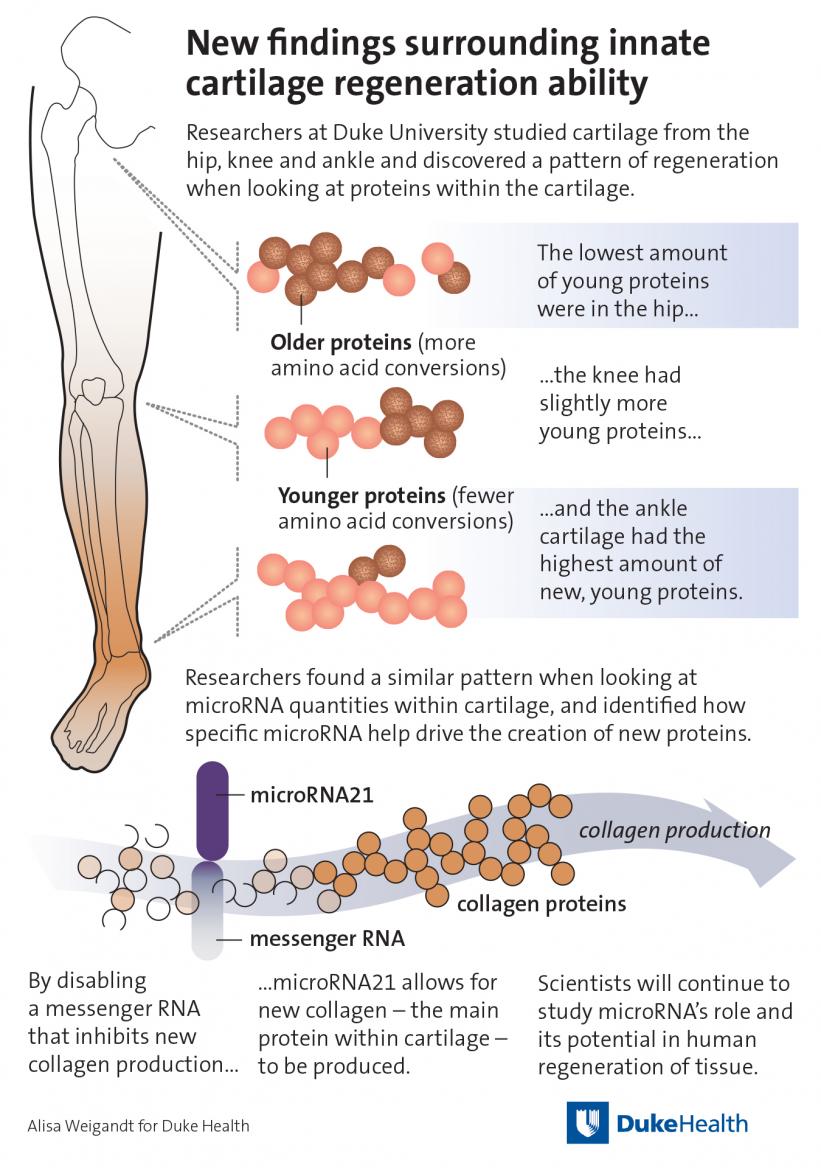
DURHAM, NC – Contrary to popular belief, cartilage in human joints can repair itself through a process similar to that used by creatures such as salamanders and zebrafish to regenerate limbs, researchers at Duke Health found.
Publish online Oct. 9 in the journal Science Advances, the researchers identified a mechanism for cartilage repair that appears to be more robust in ankle joints and less so in hips. The finding could potentially lead to treatments for osteoarthritis, the most common joint disorder in the world.
“We believe that an understanding of this ‘salamander-like’ regenerative capacity in humans, and the critically missing components of this regulatory circuit, could provide the foundation for new approaches to repair joint tissues and possibly whole human limbs,” said senior author Virginia Byers Kraus MD PhD, a professor in the departments of Medicine, Pathology and Orthopedic Surgery at Duke.
Kraus and colleagues, including lead author Ming-Feng Hsueh PhD, devised a way to determine the age of proteins using internal molecular clocks integral to amino acids, which convert one form to another with predictable regularity.
Newly created proteins in tissue have few or no amino acid conversions; older proteins have many. Understanding this process enabled the researchers to use sensitive mass spectrometry to identify when key proteins in human cartilage, including collagens, were young, middle-aged or old.
They found that the age of cartilage largely depended on where it resided in the body. Cartilage in ankles is young, it’s middle-aged in the knee and old in the hips. This correlation between the age of human cartilage and its location in the body aligns with how limb repair occurs in certain animals, which more readily regenerate at the furthest tips, including the ends of legs or tails.
The finding also helps explain why injuries to people’s knees and, especially, hips take a long time to recover and often develop into arthritis, while ankle injuries heal quicker and less often become severely arthritic.
The researchers further learned that molecules called microRNA regulate this process. Not surprisingly, these microRNAs are more active in animals that are known for limb, fin or tail repair, including salamanders, zebrafish, African fresh water fish and lizards.
These microRNAs are also found in humans — an evolutionary artifact that provides the capability in humans for joint tissue repair. As in animals, microRNA activity varies significantly by its location: it was highest in ankles compared to knees and hips and higher in the top layer of cartilage compared to deeper layers of cartilage.
“We were excited to learn that the regulators of regeneration in the salamander limb appear to also be the controllers of joint tissue repair in the human limb,” Hsueh said. “We call it our inner salamander capacity.”
The researchers said microRNAs could be developed as medicines that might prevent, slow or reverse arthritis.
“We believe we could boost these regulators to fully regenerate degenerated cartilage of an arthritic joint. If we can figure out what regulators we are missing compared with salamanders, we might even be able to add the missing components back and develop a way someday to regenerate part or all of an injured human limb,” Kraus said. “We believe this is a fundamental mechanism of repair that could be applied to many tissues, not just cartilage.
 |
| In addition to Kraus and Hsueh, study authors include Patrik Önnerfjord, Michael. P. Bolognesi and Mark. E. Easley. The study received support from an OARSI Collaborative Scholarship, a Collaborative Exchange Award from the Orthopaedic Research Society and the National Institutes of Health (P30-AG-028716). |
![]() Source Duke Health
Source Duke Health
| References |
Analysis of “old” proteins unmasks dynamic gradient of cartilage turnover in human limbs, Ming-Feng Hsueh, Patrik Önnerfjord, Michael P. Bolognesi, Mark E. Easley, Virginia B. Kraus. Science Advances. 09 Oct 2019: Vol. 5, no. 10, eaax3203. DOI: 10.1126/sciadv.aax3203. Full text
| Further reading |
Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation, Haraden CA, Huebner JL, Hsueh MF, Li YJ, Kraus VB. Arthritis Res Ther. 2019 Jun 13;21(1):146. doi: 10.1186/s13075-019-1923-x. Full text
Predictive Validity of Radiographic Trabecular Bone Texture in Knee Osteoarthritis: The Osteoarthritis Research Society International/Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium, Kraus VB, Collins JE, Charles HC, Pieper CF, Whitley L, Losina E, Nevitt M, Hoffmann S, Roemer F, Guermazi A, Hunter DJ; OA Biomarkers Consortium. Arthritis Rheumatol. 2018 Jan;70(1):80-87. doi: 10.1002/art.40348. Epub 2017 Dec 15. Full text
Establishment of reference intervals for osteoarthritis-related soluble biomarkers: the FNIH/OARSI OA Biomarkers Consortium, Kraus VB, Hargrove DE, Hunter DJ, Renner JB, Jordan JM. Ann Rheum Dis. 2017 Jan;76(1):179-185. doi: 10.1136/annrheumdis-2016-209253. Epub 2016 Jun 24.
Cartilage biomarkers in the osteoarthropathy of alkaptonuria reveal low turnover and accelerated ageing, Taylor AM, Hsueh MF, Ranganath LR, Gallagher JA, Dillon JP, Huebner JL, Catterall JB, Kraus VB. Rheumatology (Oxford). 2017 Jan;56(1):156-164. doi: 10.1093/rheumatology/kew355. Epub 2016 Oct 7. Full text
Cartilage matrix remodelling differs by disease state and joint type, Hsueh MF, Kraus VB, Önnerfjord P. Eur Cell Mater. 2017 Aug 24;34:70-82. doi: 10.22203/eCM.v034a05. Full text
Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium, Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, Wang SX, Sandell LJ, Hoffmann SC, Hunter DJ; OA Biomarkers Consortium. Ann Rheum Dis. 2017 Jan;76(1):186-195. doi: 10.1136/annrheumdis-2016-209252. Epub 2016 Jun 13. Full text
Elucidating the Molecular Composition of Cartilage by Proteomics, Hsueh MF, Khabut A, Kjellström S, Önnerfjord P, Kraus VB. J Proteome Res. 2016 Feb 5;15(2):374-88. doi: 10.1021/acs.jproteome.5b00946. Epub 2016 Jan 15. Full text
Biomarkers and proteomic analysis of osteoarthritis, Hsueh MF, Önnerfjord P, Kraus VB. Matrix Biol. 2014 Oct;39:56-66. doi: 10.1016/j.matbio.2014.08.012. Epub 2014 Aug 30. Review. Full text
Cells keep a memory of their tissue origin during axolotl limb regeneration, Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Nature. 2009 Jul 2;460(7251):60-5. doi: 10.1038/nature08152.
Also see
Science discovers we have an ‘inner salamander’ and can regrow cartilage in our joints in The National Post
